Dr Peter Sjöstedt-Hughes, Lecturer at Exeter University, has proposed incorporating metaphysical philosophy into psychedelic therapy to help improve therapeutic outcomes.
Sjöstedt-Hughes suggests that psychedelic therapy may gain more advantage by extending its scope into metaphysics, helping patients better integrate and understand psychedelic-induced metaphysical experiences.
Such improved outcomes may be seen if patients undergoing this therapy “are provided with an optional, additional, and intelligible schema and discussion of metaphysical options at the integrative phase of the therapy.”
In the paper, Sjöstedt-Hughes puts forward this schema as the “Metaphysics Matrix” and an accompanying “Metaphysics Matrix Questionnaire (MMQ)” which can be utilised by therapists and researchers as a tool for the quantitative measurement of a psychedelic experience.
The paper ‘On the need for metaphysics in psychedelic therapy and research’ has been published in Frontiers in Psychology.
What is metaphysics?
While mysticism deals with understanding the universe through direct experience, such as revelation, metaphysics is a branch of philosophy that deals with understanding the fundamental nature of reality through logic/argument.
Sjöstedt-Hughes writes that “metaphysics is not mysticism” but there is overlap: “[…] metaphysics is broader and its positions can be logically deliberated — as such metaphysics can encompass mystical experiences induced by psychedelic intake yet metaphysics can also ground those experiences in a manner that can be more intelligible, comprehensive, viable, and acceptable to participants than that which the framework of mysticism alone can offer.”
The Metaphysics Matrix
A number of clinical trials investigating psychedelic-assisted psychotherapy for the treatment of mental health conditions, such as anxiety and depression, report that participants who undergo a “mystical experience” during a psychedelic session often have higher levels of sustained therapeutic outcomes.
In clinical trials, mystical experiences are measured by different scales including the Mystical Experience Questionnaire (MEQ), the Hood Mysticism Scale (HMS), the Hallucinogen Rating Scale (HRS), the Five Dimensions Altered State of Consciousness Questionnaires (5D-ASC) and Eleven Dimensions Altered State of Consciousness Questionnaires (11D-ASC).
Sjöstedt-Hughes writes: “Data derived in this manner is obviously limited and abstract not only because psychedelic experience need not be “mystical,” but also because the definition of “mystical” could be expanded to include other criteria […]
“With regard to psychedelic-assisted psychotherapy […] speaking about mystical experience per se will not be sufficient to provide a meaningful explanation of the significance of such experience to a person, for the simple reason that mystical experience is the phenomenon to be explained — mystical experience is the explanandum rather than the explanation.
“It is metaphysics that is the means of explanation, the explanans of the mystical explanandum.”
The Metaphysics Matrix has been designed to provide a “menu” of metaphysical options that may help people to “frame, make sense of, and give significance to, their experiences”, and would be another tool in the belt of therapists to better understand these experiences.
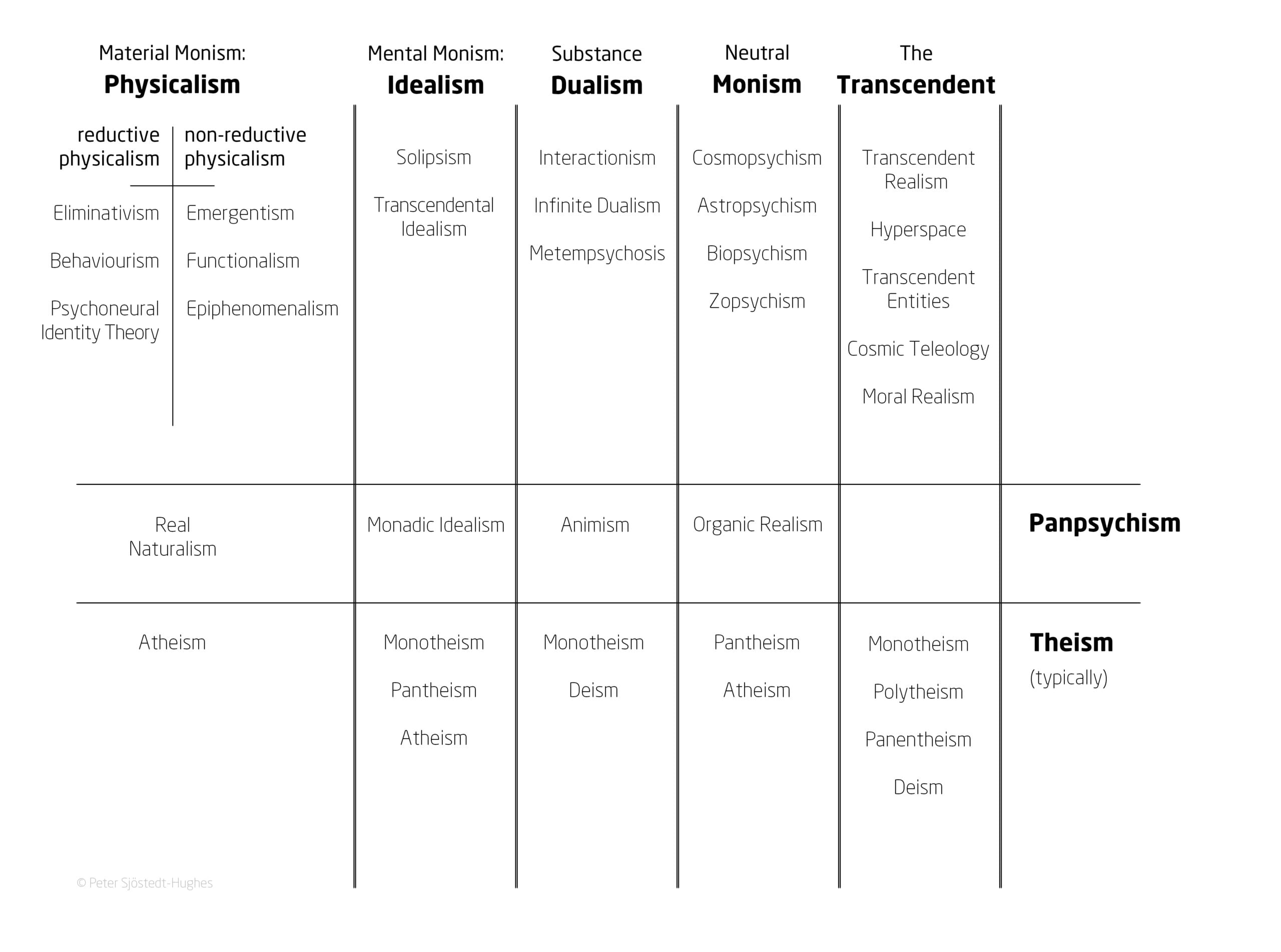
Image provided by Dr Peter Sjöstedt-Hughes.
Such experiences could be understood through metaphysical systems such as Neutral Monism, Pantheism, Panpsychism, Animism, Substance Dualism, and Idealism, says Sjöstedt-Hughes.
Some examples provided include the common experience of the Universe being God – which can be understood in the context of Pantheism – or of all matter having a basic form of sentience – such as plants having a basic drive or process – which can be understood in the context of Panpsychism.

Image provided by Dr Peter Sjöstedt-Hughes.
Additionally, enabling people who have had these experiences to understand them within these frameworks may make them less likely to dismiss the experiences as delusional, says Sjöstedt-Hughes.
“ […] Relatedly, that the worldview hitherto adopted by the participant is but one metaphysical position amongst others,” he writes.
Sjöstedt-Hughes commented: “This is a conjecture that hasn’t been tested but can be tested – offering a patient an additional and optional discussion in the integrative phase of psychedelic-assisted psychotherapy.
“Giving them this Metaphysics Menu for integration may extend the long-term benefits of psychedelic therapy and beyond because there’s a number of studies that seem to show that certain peak psychedelic experiences have the longest and most beneficial health outputs results.
“If in the integrative phase [of therapy] one looks at that experience and starts to frame it intelligibly, then the conjecture is that the participant will not in a few weeks after that, think it must have been a delusion – they will say that we don’t know what reality is.
“Therefore, we can’t dismiss something as a delusion necessarily. By doing that it might extend the significance of that experience for the person.
“When we use Mysticism Scales, by definition, mystery can’t explain itself. Metaphysics, however, incorporates those experiences and offers an explanation to what they mean. For example, the relation between oneself and the universe.”
Sjöstedt-Hughes points out that in practice, one of the immediate issues is the practical issue of implementation of Metaphysics Integration, suggesting this could be supported through resources such as a handbook or practitioner training.
He further concludes the integration would need to be “further bridged by the therapist to the participant’s life, concerns, values, aims, and outlook.”
The Metaphysics Schema is already being utilised in studies taking place at Ohio State University, US, and Exeter University, UK.

 Opinion2 years ago
Opinion2 years ago
 Insight3 years ago
Insight3 years ago
 Research2 years ago
Research2 years ago
 Medicinal2 years ago
Medicinal2 years ago
 Markets & Industry1 year ago
Markets & Industry1 year ago
 News3 years ago
News3 years ago
 Medicinal2 years ago
Medicinal2 years ago
 Research2 years ago
Research2 years ago